Crude
Oil
Northeastern
Utah field (

Upper

Panhandle
region, Texas (USA)
Southwestern
Illinois, (

Characteristics and Hazards of Crude Oil
Petroleum,
or crude oil, is a naturally occurring oily, bituminous liquid composed of
various organic chemicals.
It is
found in large quantities below the surface of the earth and is used as a fuel
and as a raw material in the chemical industry.
Petroleum
is formed under the earth's surface by the decomposition of organic material.
Organic
material arising out of:
These
combined with the fine sands and silts in calm sea basins went to form the vast
reservoirs of crude oil. This process began many millions of years ago with the
development of abundant life, and it continues to this day. The sediments grow
thicker and sink into the sea floor under its own weight.
As
additional deposits pile up, the pressure on the ones below increases several
thousand times, and the temperature rises by several hundred degrees. The mud and sand harden into shale and
sandstone. Carbonate precipitates and skeletal shells harden into
limestone.
The
remains of the dead organisms are then transformed into crude oil and natural
gas.
The
range and complexity of naturally occurring petroleum is extremely large and
the variation in composition from one reservoir to another shows quite a range.
Crude
Oil
Northeastern
Utah field (

Upper

Panhandle
region, Texas (USA)


Southwestern
Illinois, (

Crude
oil is graded by a specific viscosity range indicated as degrees API (American
Petroleum Institute) gravity.
The
higher the API gravity, the lighter (low specific gravity) would be the crude.
For
example, light crude oils have high API gravity's and low specific gravity.
All
crude oils differ in the fractions of the various hydrocarbons they contain.
The
specific molecules vary in shape and size from (Carbon) C1 to C80 or more.
At
the simplest, the one hydrocarbon compound has four hydrogen atoms bonded to
the carbon atom to produce a compound CH4, or methane gas.
Crude
oils with low carbon, high hydrogen and high API gravity are usually rich in
paraffin's and tend to yield greater proportions of gasoline and light
petroleum products; whereas those with high carbon, low hydrogen, and low API
gravities are usually rich in aromatics.
Crude
oils that contain appreciable quantities of hydrogen sulfide or other reactive
sulfur compounds are called "sour." Those with less sulfur are called
"sweet."
Some
exceptions to this rule are
Liquid
hydrocarbons from natural wells may have nitrogen, oxygen, and sulfur in
quantities from trace amounts to significant, as well as traces of metals.
Three broad classes of crude
petroleum exist:
The
paraffin types are composed of molecules in which the number of hydrogen atoms
is always two more than twice the number of carbon atoms. (2n+2)
The
characteristic molecules in the asphaltic types are naphthenes, composed of
twice as many hydrogen atoms as carbon atoms.
In
the mixed-base group are both paraffin hydrocarbons and naphthenes.
Paraffin’s
The
saturated open-chain hydrocarbons form a homologous series called the paraffin
series or the alkane series.
The
composition of each of the members of the series corresponds to the formula
CnH2n + 2, where n is the number of carbon atoms in the molecule.
All
the members of the series are un-reactive.
They do not react readily at ordinary temperatures with such reagents as
acids, alkalis, or oxidizers.
The
first four carbon molecules, C1 to C4, with the addition of hydrogen, form
hydrocarbon gases:
Larger
molecules C5 to C7 cover the range of light gasoline liquids;
Accompanying
the gas compounds may be various amounts of nitrogen, carbon dioxide, hydrogen
sulfide, and occasionally helium.
Alkene Series
The
unsaturated open-chain hydrocarbons include the alkene or olefin series, the
diene series, and the alkyne series.
The
alkene series is made up of chain hydrocarbons in which a double bond exists
between two carbon atoms.
The
general formula for the series is CnH2n, where n is the number of carbon
atoms.
Similar
to the paraffin series, the lower members are gases, intermediate compounds are
liquids, and the higher members are solids.
The
alkene series compounds are more active chemically than the saturated
compounds. They react easily with
substances such as halogens by adding atoms at the double bonds.
They
are not found to any extent in natural products, but are produced in the
destructive distillation of complex natural substances, such as coal, and are
formed in large amounts in petroleum refining, particularly in the cracking
process.
The
first member of the series is ethylene, C2H4.
Dienes
Contain
two double bonds between pairs of carbon atoms in the molecule. They are related to the complex hydrocarbons
in natural rubber and are important in the manufacture of synthetic rubber and
plastics. The most important members of
this series are butadiene, C4H6 and isoprene, C5H8.
Alkyne Series
The
members of the alkyne series contain a triple bond between two carbon atoms in
the molecule. They are very active
chemically and are not found free in nature.
The
first and most important member of the series is acetylene, C2H2
Cyclic Hydrocarbons
The
simplest of the saturated cyclic hydrocarbons, or cycloalkanes, is
cyclopropane, C3H6, the molecules of which are made up of three carbon atoms to
each of which two hydrogen atoms are attached.
Cyclopropane is somewhat more reactive than the corresponding open-chain
alkane, propane, C3H8.
Other
cycloalkanes make up a part of ordinary gasoline. The most important group of
unsaturated cyclic hydrocarbons is the aromatics, which occur in coal tar. The aromatics sometimes exhibit unsaturation,
that is, the addition of other substances, their principal reactions bring
about the replacement of hydrogen atoms by other kinds of atoms or groups of
atoms.
The
aromatic hydrocarbons include benzene, toluene, anthracene, and naphthalene.
Natural
Gas occurs in mixtures of hydrocarbon gases and vapors, the more important of
which are:
Natural
Gas is lighter than air, non-toxic and contains no poisonous ingredients.
Breathing
natural gas is harmful when there is not an adequate supply of oxygen in the
atmosphere.
Properties
Methane (C1)
Methane,
also referred to as marsh gas, is a gas composed of carbon and hydrogen with a
chemical formula of CH4. It is lighter than air, colorless, odorless, tasteless
and is flammable. It occurs in natural
gas and as a by-product of petroleum refining.
Propane (C3)
Propane
is a colorless, odorless gas of the alkane series of hydrocarbons, of formula
C3H8.
It
occurs in crude oil, natural gas, and as a by-product of refinery cracking gas
during petroleum refining. Propane does
not react strongly at room temperature.
It does react, however, with chlorine at room temperature if the mixture
is exposed to light.
Butane (C4)
Butane,
is the either of two saturated hydrocarbons, or alkanes, with the chemical
formula of C4H10 of the paraffin series.
In both compounds the carbon atoms are joined in an open chain. In n-butane (normal), the chain is continuous
and un-branched whereas in i-butane (iso) one of the carbon atoms forms a side
branch.
LPG, Liquefied Petroleum Gas
(C3 & C4)
Liquefied
Petroleum Gas (LPG) is a mixture of the liquefied gases of propane (C3) and
butane (C4).
It is
obtained from natural gas or petroleum.
It
has a flammability range of 1.8% to 10% and the vapor has a density of 1.5 to
2.0 that of air.
One
volume of LPG liquid may form 2,300 to 13,500 time the volume of gas in
air.
LPG
vapor is an anesthetic and asphyxiant in high concentrations.
Gasoline
(C5 to C11)
Commercial
gasolines are a mixture of straight -run, cracked, reformed, and natural
gasolines.
Gasoline
is a mixture of the lighter liquid hydrocarbons that distills within the range
of 38 to 204 ºC (100 to 400 ºF).
The
yield of gasoline from this process varies from, about 1 percent to about 50
percent, depending on the petroleum.
Condensate (C4, C5, C6 & C
- higher)
Condensate
is normally considered the entrapped liquids in process or production gas
streams due to temperature or pressure, in the typically in the range of C3,
C4, C5 or heavier hydrocarbon liquids.
Kerosene
Kerosene
or sometimes referred to as Fuel Oil # 1- is a refined petroleum
distillate.
Kerosene’s
usually have flash points within the range of 37.8 °C to 54.4 °C (100 °F to 130 °F).
Therefore
unless heated, kerosene will usually not produce ignitable mixtures over its
surface.
Diesel
Diesel
or sometimes referred to Fuel Oil #2 is the fraction of petroleum that distills
after kerosene;
This
is considered to be in the family of gas oils.
Lubricating Oils and Greases
(C20 to C27)
Vacuum
distillates or residual fraction of vacuum distillates are the main source of
lubricating oils from the petroleum industry.
Grease
Grease
is a thick, oily, lubricating material that typically has a smooth, spongy or
buttery feel.
Lubricating
greases are made by thickening lubricating oils with soaps, clays, silica gel
or other thickening agents. Greases
range from soft semi-fluids to hard solids, the hardness increasing as the
content of the thickening agent increases.
Asphalt and Waxes (C28 & C-
higher)
Asphalt
is a bituminous substance that is found in natural deposits or as the residual
of in petroleum or coal tar refining processes.
It
has a black or brownish-black color and pitchy luster.
It is
cement-like in nature varying in consistency at room temperature from solid to
semisolid depending on the amount of light hydrocarbon fractions that have been
removed.
Wax
Wax is
a soft impressionable semi-solid material having a dull luster and a somewhat
soapy or greasy texture. It softens
gradually upon heating, going through a soft, malleable state before ultimately
forming a liquid. Paraffin wax is a
mixture of saturated hydrocarbons of higher molecular mass, produced during the
refining of petroleum.
Gas and Fuel Oils (CI2 to C19)
Gas
oil or fuels oil is a generic term applied to petroleum distillates boiling
between kerosene and lubricating oils.
The name gas oil was originally derived from its initial use for making
illuminating gas, but is now used as a burner fuel, diesel engine fuel, and
catalytic cracker charge stock.
Gas
oils contain fuel oils such as kerosene, diesel fuels, gas turbine fuels, etc.
Non hydrocarbons that are
found in crude oil:
Products obtained from the
refining process:
Hazard- Health
Hydrocarbon
materials have several different characteristics that can be used to define
their level of hazard. Since no one
feature can adequately define the level of risk for a particular substance they
should be evaluated as a synergism. It
should also be realized that these characteristics have been tested under
strict laboratory conditions and procedures that may alter when
applied to industrial environments.
The toxic hazards
to which ships personnel are exposed during operations and carriage arise
almost entirely from exposure to gases of various kinds.
Generally nearly
all substances have been assigned:
The term Threshold
Limit Value (TLV) is often expressed as a time weighted Average (TWA).
The use of the
term Permissible Exposure Limit refers to the maximum exposure to a toxic
substance that is allowed by an appropriate regulatory body.
The PEL is usually
expressed as a Time Weighted Average, normally averaged over an eight-hour
period
Short Term
Exposure Limit (STEL), is normally expressed as a maximum airborne
concentration averaged over a 15-minute period.
The values are
expressed as parts per million (PPM) by volume of gas in air.
Toxicity can be
greatly influenced by the presence of some minor components such as aromatic
hydrocarbons (e.g. benzene) and hydrogen sulphide.
A TLV of 300PPM,
corresponding to about 2%LEL, is established for gasoline vapours.
Concentration %LEL Effects
0.1% vol. (1,000 PPM) 10% Irritation of the eyes within
one hour.
0.2% vol. (2,000 PPM) 20% Irritation of the eyes, nose and throat, dizziness
and unsteadiness within half an hour.
0.7% vol. (7,000 PPM) 70% Symptoms as of drunkenness
within 15 minutes.
1.0% vol. (10,000 PPM) 100% Rapid
onset of ‘drunkenness’, which may lead to unconsciousness and death if exposure
continues.
2.0% vol. (20,000 PPM) 200% Paralysis and death occur very
rapidly.
Typical effects of exposure
to petroleum gases
The smell of
petroleum gas mixtures is very variable, and in some cases the gases may dull
the sense of smell.
The impairment of
smell is especially serious if the mixture contains hydrogen sulphide.
The absence of
smell should therefore never be taken to indicate the absence of gas.
The TLV concentration is considerably below the lower
flammable limit and combustible gas indicators cannot be expected to measure
concentrations of this order accurately
The aromatic
hydrocarbons include benzene,
toluene and xylene. These substances are components in varying amounts, in
many typical cargoes. The health hazard of aromatic hydrocarbons is not fully
established but it is recommended that personnel engaged in cargo operations
involving products containing them follow the precautions and procedures.
The Threshold
Limit Value (TLV) or Permissible Exposure Limit (PEL), of an aromatic
hydrocarbon vapour is generally less than that of other hydrocarbons
Repeated over
exposure to high levels of Benzene vapour may have chronic effects, which can
lead to disorders of the blood and bone marrow
Benzene primarily
presents an inhalation hazard.
It has poor
warning qualities, as its odour threshold is well above the Permissible
Exposure Limit.
Exposure to
concentrations in excess of 1,000 PPM can lead to unconsciousness and even
death.
Benzene can also
be absorbed through the skin and is toxic if ingested
HYDROGEN
SULPHIDE
The Permissible
Exposure Limit (PEL) of hydrogen sulphide expressed as a Time Weighted
Average (TWA) is
10 PPM.
Concentration Effects
50-100 PPM Eye
and respiratory tract irritation after exposure of one hour.
200-300 PPM Marked
eye and respiratory tract irritation after exposure of one hour.
500-700 PPM Dizziness,
headache, nausea etc. within 15 minutes, loss of consciousness and possible
death after 30-60 minutes exposure.
700-900 PPM Rapid
unconsciousness, death occurring a few minutes later.
1,000-2,000 PPM Instantaneous collapse and cessation of breathing.
Note: Persons
over exposed to H2S vapour should be removed to clean air as soon as possible.
The adverse effects of H2S can be reversed and the probability of saving the
person’s life improved if prompt action is taken.
For example a
crude oil containing 70 PPM (by weight) hydrogen sulphide has been shown to
produce a concentration of 7,000 PPM (by volume) in the gas stream leaving an
ullage port above the cargo tank.
Thus, it is not
possible to predict the likely vapour concentration from known liquid
concentrations.
Prior to entry
into a tank which has previously carried petroleum products containing hydrogen
sulphide, the tank should initially be ventilated to a reading of less than 1%
LFL on a combustible gas indicator and then checked using the appropriate
instruments to ensure that there are no detectable traces of hydrogen sulphide.
Inert gas
Inert gas
composition
Component IG
from main boiler flue gas
Nitrogen (N2) 83%
Carbon dioxide (CO2) 13%
Carbon monoxide (CO) Present
Oxygen (O2) 4%
Sulphur dioxide (SO2) 50
PPM
Oxides of Nitrogen (NOx) Present
Water Vapour (H2O) Present
Ash and Soot (C) Present
Dew point High
if not dried
Density 1.044
The main hazard
associated with inert gas is its low oxygen content; it also contains trace
amounts of various toxic gases which may increase the hazard to personnel
exposed to it.
Nitrogen
dioxide is even more toxic with a TLV of 3 PPM.
Sulphur dioxide
produces irritation of the eyes, nose and throat and may also cause breathing
difficulties in sensitive people
Carbon monoxide
is an odourless gas with a TLV of 50 PPM.
It is insidious
in its attack, which is to restrict oxygen uptake by the blood, causing a
chemically induced form of asphyxiation.
LACK OF OXYGEN
As the amount of
available oxygen decreases below the normal 21% by volume breathing tends to
become faster and deeper. Symptoms indicating that an atmosphere is deficient
in oxygen may give inadequate notice of danger. Most
persons would fail to recognise the danger until they were too weak to be able
to escape without help.
This is especially
so when escape involves the exertion of climbing.
While individuals
vary in susceptibility, all will impaired if the oxygen level falls to 16% by volume.
Exposure to an atmosphere containing less than 10% oxygen content by volume
inevitably causes unconsciousness. An atmosphere containing less than 5% oxygen
by volume causes immediate unconsciousness with no warning other that a gasp
for air.
If resuscitation
is delayed for more that a few minutes (about 4 minutes), irreversible damage
is done to the brain, even if life is subsequently restored.
Hazards - Flammability
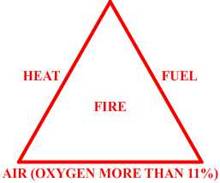
Burning/Igniting/Explosion
- all of these are related to the property of a Hydrocarbon (Hc) gas to react
with the oxygen in the air to produce carbon dioxide and water. This reaction
between the two gives off enough heat to form a flame which travels through the
above mixture.
When
the gas above the liquid Hydrocarbon (Hc) ignites and burns, sufficient heat is
generated to vaporize more liquid and the fire is thus fueled, thus it is
actually the gas which burns and the effect is seemingly the liquid which is on
fire.
This
is one reason that, a flash back may occur if dousing a fire with water; water
is a heat remover as such if sufficient quantities are not used, and the liquid
Hydrocarbon (Hc) floating on the surface of the water may again get ignited,
due to residual heat.
Flashpoint
Open cup
flashpoint.
A sample of the
liquid is gradually heated in a special pot and a small flame is repeatedly and
momentarily applied to the surface of the liquid.
The flashpoint is
the lowest liquid temperature at which the small flame initiates a flash of
flame across the surface of the liquid, thereby indicating the presence of a
flammable gas/air mixture above the liquid.
For all oils,
except some residual fuel oils, this gas/air mixture corresponds closely to the
lower flammable limit mixture.
Closed cup
flashpoint
The space above
the liquid is kept closed except for brief moments when the initiating flame is
introduced through a small port.
Because of the
greater loss of gas to atmosphere in the open cup test the open cup flashpoint
of a petroleum liquid is always a little higher (by about 6ºC) than its closed
cup flashpoint.
Non-volatile
Flashpoint of 60ºC
or above as determined by the closed cup method of testing.
These liquids
produce, when at any normal ambient temperature, equilibrium gas concentrations
below the lower flammable limit.
They include
distillate fuel oils, heavy gas oils and diesel oils.
Their (Reid Vapour
Pressure) RVPs are below 0.007 bar and are not usually measured.
Volatile
Flashpoint below
60ºC as determined by the closed cup method of testing
Some petroleum
liquids in this category are capable of producing an equilibrium gas/air
mixture within the flammable range when in some part of the normal ambient
temperature range, while most of the rest give equilibrium gas/air mixtures
above the upper flammable limit at all normal ambient temperatures.
Examples of the
former are jet fuels and kerosenes and of the latter gasolines and most crude
oils. In practice, gasolines and crude oils are frequently handled before
equilibrium conditions have been attained and gas/air mixtures in the flammable
range may then be present.
IMPORTANT
If there
is any doubt as to the characteristics of a cargo, or if a non-volatile cargo
is being handled at a temperature above its flashpoint minus 10ºC, it should be
treated as volatile petroleum.
(Example
– If loading a grade of oil (flash point of 65ºC) then it will be treated as
Volatile cargo – if the loading temperature is above 55ºC).
Owing to their
particular characteristics, residual fuel oils should always be treated as
volatile
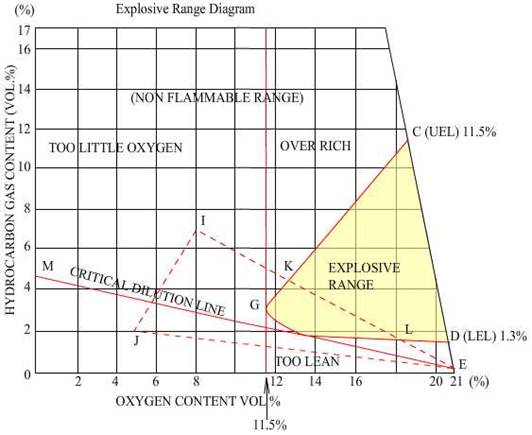
Density of Hydrocarbon Gases:
Undiluted
hydrocarbon gas is always heavier than air; it thus has a property to be dense
in layers, with those in contact with the oil being denser than those at the
boundary with air.
At
the Lower Flammable limit, the gas has a density that is
indistinguishable from air, that is, it is the nearly the same as air density.
A
hydrocarbon and air mixture cannot burn unless the composition lies within the
flammable range. This range is defined as that where the %volume of the gas in
air is just sufficient to begin combustion to a concentration where the %
volume exceeds a predetermined value where the mixture is incapable of burning.
PYROPHORIC IRON SULFIDE
A
substance typically formed inside tanks by the corrosive interaction of sulfur
compounds in the hydrocarbons and the iron and steel in the hull and
structural. On exposure to air (oxygen) it ignites spontaneously, and in
air/hydrocarbon mixture can cause an explosion.
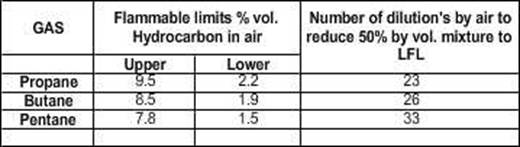
Some
common petroleum materials and their flammable limits under normal conditions
are listed below beginning with the widest ranges:
Material
Hydrogen 4.0 to 75.6 71.6
Ethane 3.0 to 15.5 12.5
Methane 5.0 to 15.0 10.0
Propane 2.0 to 9.5 7.5
Butane 1.5 to 8.5 7.0
Pentane 1.4 to 8.0 6.6
Hexane 1.7 to 7.4 5.7
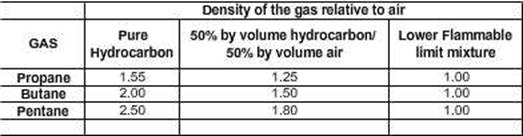
Flammable
limits, Propane, Butane, Pentane
Vapour Pressure
All crude oils and
the usual petroleum products are essentially mixtures of a wide range of
hydrocarbon compounds (i.e. chemical compounds of hydrogen and carbon).
The (Boiling
point) BP of these compounds range from -162ºC (methane) to well in excess of
+400ºC, and the volatility of any particular mixture of compounds depends
primarily on the quantities of the more volatile constituents (i.e. those with
a lower boiling point).
The volatility is characterised by the vapour pressure.
When a petroleum
mixture is transferred to a gas free tank or container it commences to
vaporise.
There is also a
tendency for this gas to re-dissolve in the liquid, and equilibrium is
ultimately reached with a certain amount of gas evenly distributed throughout
the space.
The pressure exerted
by this gas is called the equilibrium vapour pressure of the liquid, usually
referred to simply as the vapour pressure.
The vapour
pressure of a pure compound depends only upon its temperature.
The vapour
pressure of a mixture depends on its temperature, constituents and the volume
of the gas space in which vaporisation occurs.
The True Vapour
Pressure (TVP) or bubble point vapour pressure is the equilibrium vapour
pressure of a mixture when the gas/liquid ratio is effectively zero.
It is the highest
vapour pressure which is possible at any specified temperature.
As the temperature
of a petroleum mixture increases its TVP also increases.
If the TVP exceeds
atmospheric pressure the liquid commences to boil.
The TVP of a
petroleum mixture provides a good indication of its ability to give rise to
gas.
Reid Vapour
Pressure
A sample of the
liquid is introduced into the test container at atmospheric pressure so that
the volume of the liquid is one fifth of the total internal volume of the
container.
The container is
sealed and immersed in a water bath where it is heated to 37.8ºC.
After the
container has been shaken to bring about equilibrium conditions rapidly, the
rise in pressure due to vaporisation is read on an attached pressure gauge.
This pressure
gauge reading gives a close approximation, in bars, to the vapour pressure of
the liquid at 37.8ºC.
RVP is useful for
comparing the volatilities of a wide range of petroleum liquids in a general
way.
It is, however, of
little value in itself as a means of estimating the likely gas evolution in
specific situations, mainly because the measurement is made at the standard
temperature of 37.8ºC and at a fixed gas/liquid ratio. For this purpose TVP is
much more useful; as already mentioned, in some cases correlations exist
between TVP, RVP and temperature.